Experimental approaches
The use of microwaves in biomass pretreatment
- Microwaves are electromagnetic energy waves with wavelengths from 1 m to 1 mm and frequencies between 0.3 – 300 GHz.
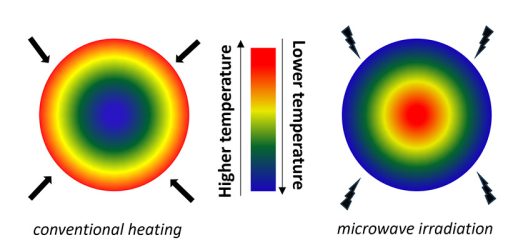
- During transmission, microwaves are transformed into suitable frequencies from which energy can be absorbed by the material being heated.
- In contrast to that in conventional heating, energy conversion in microwave irradiation results directly in volumetric heat generation within the target material and not through the material surface.
- Microwave heating has been successfully applied in biomass pretreatments where in addition to the heating process it causes swelling and fragmentation. It affects the cellulose surface area and causes decrease of crystallinity, while it promotes removal of lignin.
Heterologous expression of proteins in Pichia pastoris

- Τhe methylotrophic yeast P. pastoris is a well-established eukaryotic host for the production of heterologous proteins preferentially secreted into the medium to simplify further down-stream procedures.
- Ιts intracellular environment is generally more suitable for correct folding of eukaryotic proteins and it has the ability of post-translational disulfide bond formation, proteolytic processing and processing of signal sequences, disulfide bridge formation, certain types of lipid addition, and 0– and N-linked glycosylation, which may be crucial for biological activity.
- Τhe stable integration of expression plasmids at specific sites in P. pastoris genome, and the simplicity of techniques needed for the molecular genetic manipulation render it a promiscuous candidate for heterologous protein expression.
Immobilized Affinity Chromatography (IMAC) for the purification of the recombinant proteins

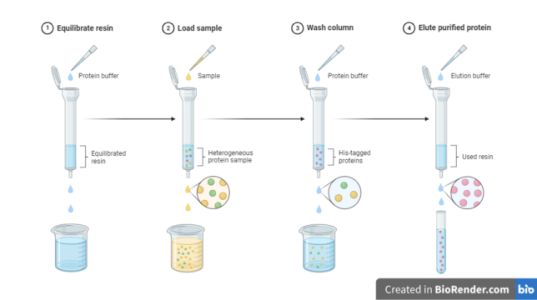
- The principle of IMAC is based on the reversible interaction between various amino acid side chains and immobilized metal ions.
- Depending on the immobilized metal ion, different side chains can be involved in the adsorption process. Most notably, histidine, cysteine, and tryptophan side chains have been implicated in protein binding to immobilized transition metal ions and zinc.
- Nikel or cobalt-based IMAC resins are designed to purify recombinant polyhistidine-tagged (His-tag) proteins and are compatible with many commonly used reagents and allows protein purification under native or denaturing conditions.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for the identification of proteins’ molecular weight


- The most commonly used technology to obtain high resolution analytical separation of mixtures of proteins is sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
- The procedure involves initial denaturation of component proteins with an anionic detergent that also binds to them, imparting to all proteins a negative charge proportional to their molecular mass. This step is followed by electrophoresis through a porous acrylamide gel matrix that separates proteins with excellent resolution on the basis of molecular mass.
Spectrophotometry for the detection of enzymes’ activity on different substrates

- Spectrophotometry is a technique used to measure the amount of light absorbed by a sample as a function of its wavelength.
- It is based on the principle that different compounds absorb light at specific wavelengths. By measuring the intensity of light before and after it passes through a sample, the amount of light absorbed by the sample can be determined.
- Spectrophotometry is commonly used to determine the concentration of a solute in a solution using Beer-Lambert’s Law, which relates absorbance to concentration.
- It is also used to monitor the progress of chemical reactions by measuring changes in absorbance over time.
High-performance liquid chromatography (HPLC) detection of enzymes’ activity on furans and the evaluation of products profile


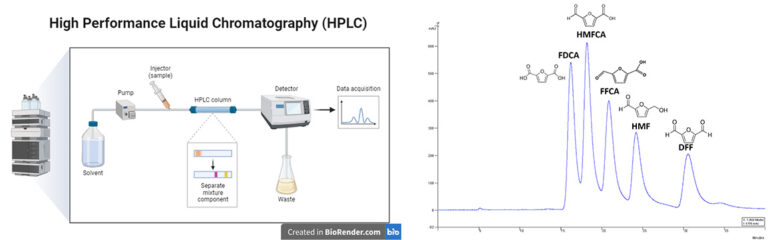
- HPLC is an advanced technique in analytical chemistry used to separate, identify, and quantify components in a mixture.
- This technique utilizes a degas unit and dual pumps to move a solvent, termed the “mobile phase” (e.g., water, acetonitrile, methanol), and the sample mixture through a column containing a “stationary phase” made of solid adsorbent materials (e.g., silica, polymers). Each sample component interacts uniquely with the adsorbent, leading to different flow rates and consequently separating the components as they exit the column.
- Analytical HPLC typically handles small sample volumes (10 to 100 µl) and utilizes columns with narrow diameters (2.1–4.6 mm) and varying lengths (30–250 mm), packed with fine adsorbent particles (2–50 µm). This provides high resolving power, making HPLC highly efficient for separating intricate mixtures.
- Temperature control, via a column oven, plays a crucial role in the separation process by affecting the interactions between sample components and the adsorbent. These interactions define each compound’s retention time, which is the duration it takes to elute from the column. Detectors like UV/Vis and refractive index detectors (RID) produce signals proportional to the amount of each sample component, enabling quantitative analysis.
